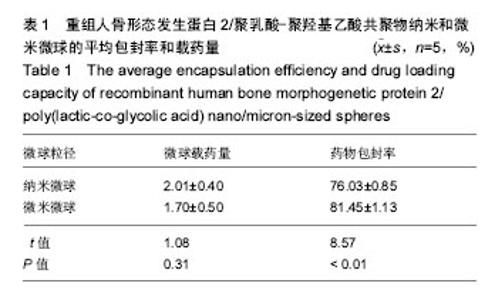
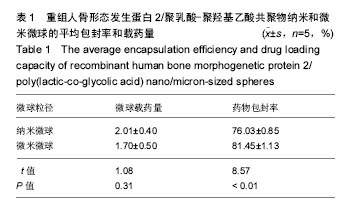
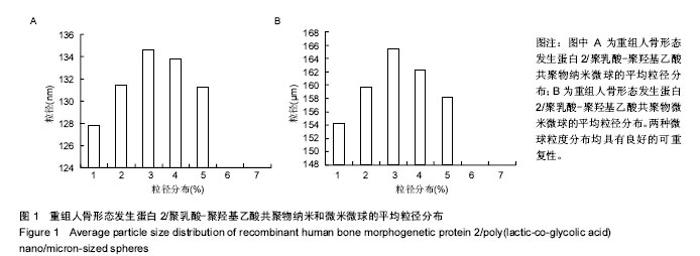
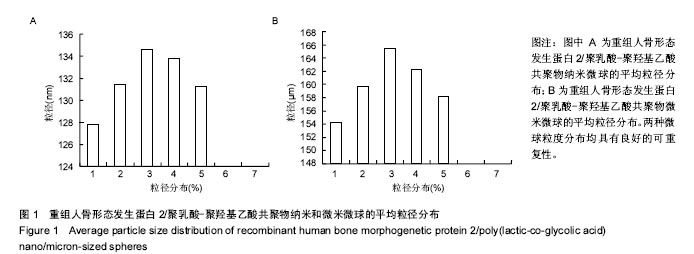
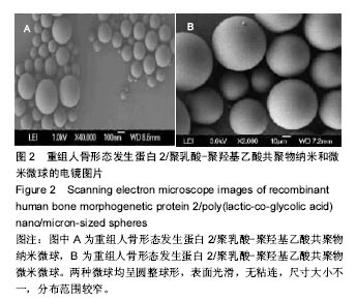
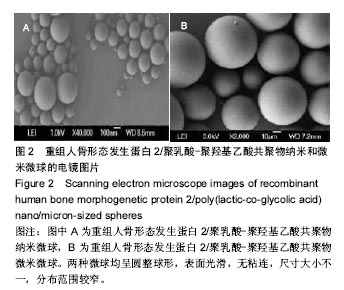
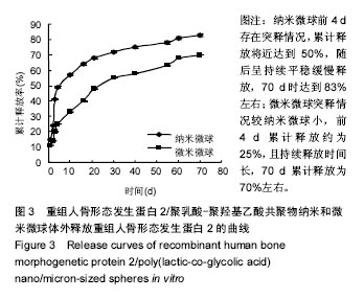
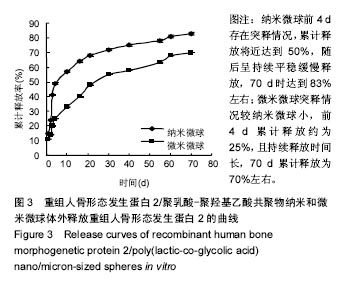
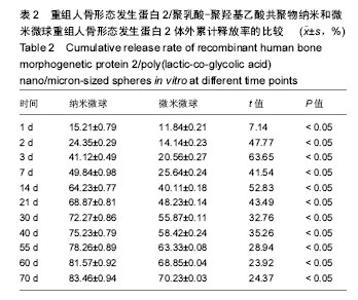
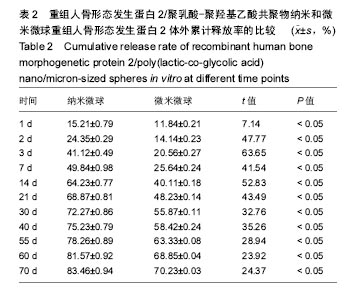
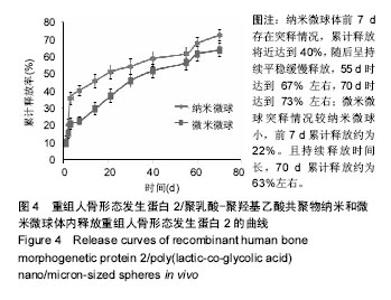
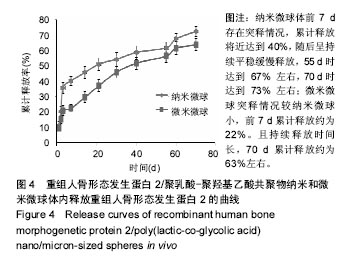
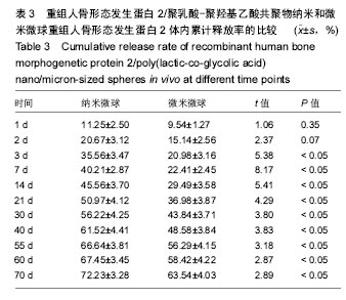
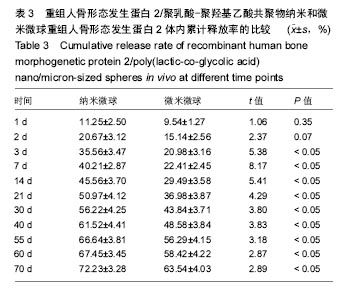
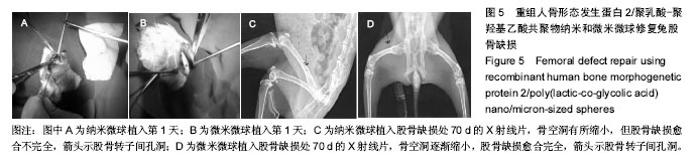
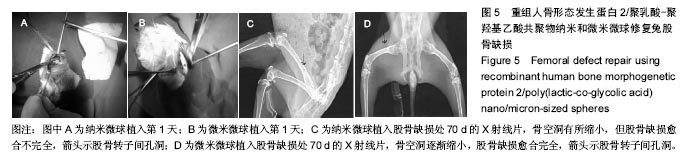
| [1]卢晓郎,郑亦静,程涛.等.骨形态发生蛋白缓释微球结合富血小板凝胶对骨髓基质干细胞增殖分化的影响[J].中国组织工程研究, 2016, 20(47):7057-7063.[2]Marsell R,Einhorn TA.The biology of fracture healing.Injury, 2015;42(6):551-555.[3]Knight DA,Rossi FM,Hackett TL.Mesenchymal stem cells for repair of the airway epithelium in asthma.Expert Rev Respir Med.2010;4(6):747-758.[4]丁琛,刘浩.缓释重组人骨形态发生蛋白 2 仿生纳米纤维肝素/明胶复合支架的制备及表征[J].中国组织工程研究, 2016,20(10): 2384-2390. [5]Haider A,Kim S,Huh MW,et al.BMP-2 Grafted nHA/PLGA Hybrid Nanofiber Scaffold Stimulates Osteoblastic Cells Growth.Biomed Res Int.2015;28(3):1909-1911. [6]刘峰,孙健,李亚莉,等.骨形态发生蛋白2壳聚糖纳米缓释载体的制备及性能测定[J].上海口腔医学,2015,24 (2):395-400. [7]唐智明,熊龙,曾建华,等.中空羟基磷灰石复合重组人骨形态发生蛋白2微球体修复骨缺损[J].中国组织工程研究, 2017,21(2):177-181. [8]向柄彦,郭元.载药微球联合BMP-2治疗兔慢性骨髓炎的实验研究[J].重庆医学,2015,44(8):1032-1036[9]巴格那,陈华荣,李婷,等.注射型纳米壳聚糖骨形态发生蛋白2 复合体诱导牙周组织的再生[J].中国组织工程研究, 2015,19(38): 6184-6190.[10]Zhao W,Li J,Jin K.Fabrication of functional PLGA-based electros pun scaffolds and their applications in biomedical engineering Mater.Sci Eng C Mater Biol Appl.2016;59: 1181-1194. [11]罗菲,卢来春,张纲.rhBMP-2-mPEG-PLA纳米缓释微球缓释凝胶的制备及体外释放实验研究[J].口腔医学, 2014,31(1):35-38.[12]费正奇,胡蕴玉,吴道澄,等.rhBMP-2/聚乳酸与聚乙醇酸共聚物载药微球的制备及成骨活性研究[J].生物医学工程与临床, 2006,10(3): 121-125.[13]Cui HX,Guo J,Han Z.Biocompatibility of differently proportioned HA/PLGA/BMP-2 composite biomaterials in rabbits.Genet Mol Res.2015;14(4):13511-13518.[14]安森博,汪龙,胡懿郃.聚乳酸-羟基乙酸共聚物载药微球性质及缓释行为的影响因素[J].中国医院药学杂志2016,36(13):1140-1144.[15]周庆梅,孙健,李亚莉,等.生长因子缓释系统复合纳米支架料修复犬下颌骨缺损[J].中国组织工程研究,2013,17(21):3815-3822.[16]黄鑫,孟国林,刘建,等.rhBMP-2壳聚糖微球的制备及体外检测[J].中国矫形外科杂志,2009,17(15):1172-1174.[17]Wen J,Kim GJ,Leong KW.Poly(D,L-lactide-co-ethyl ethylene phosphate)s as new drug carriers.J Control Release. 2003; 92(1-2):39-48. [18]Chen L,Liu L,Li C,et al.A new growth factor controlled drug release system to promote healing of bone fractures: nanospheres of recombinant human bone morphogenetic-2 and polylacticacid.J Nanosci Nanotechnol. 2011;11(4):3107-3114. [19]Carreira AC,Lojudice FH,Halcsik E,et al.Bone morphogenetic proteins: facts, challenges, and future perspectives.J Dent Res.2014;93(4):335-345.[20]Ji Y,Xu GP,Zhang ZP.BMP-2/PLGA delayed-release microspheres composite graft, selection of bone particulate diameters, and prevention of aseptic inflammation for bone tissue engineering. Ann Biomed Eng.2010;38(3):632-639. |